Patient-centric medical services-distance from traditional to smart medical device management
Chen Rei Hong, Dept. of Material Supply
Chu Yu Hui, Administrator, Dept. of Material Supply
Since the fourth industrial revolution, medical devices have been changing, drugs are constantly upgraded, and relevant technologies have undergone rapid development. Today, smart healthcare has become mainstream in the new era. In order to ensure the safety, performance, and quality of medical devices used by Taiwanese, the Ministry of Health and Welfare promulgated the Medical Devices Act on 1 May 2021 to stipulate that medical device manufacturers and medical institutions should create and store (including declaration) of information on medical device source and flow for 202 medical devices (grades 2 and 3 implantable medical devices). For example, source and flow management are required for common knee/hip implants in the operating theater and pacemakers in the cardiac catheterization room. Therefore, effective use of technology to strengthen medical device management and decrease the effort required by medical logistics staff to maintain medical quality are urgent issues.
The Material Supply Room introduced the “stage 1 automated recognition application system) in 2020 in which dedicated staff creates and records batch number/expiry date (PI) on the medical device GTIN code (DI) database during check and acceptance operations. This system was activated and used by the cardiac catheterization room and operating theater. Medical declaration and follow-up management are carried out after cross-comparison of data from the MIS inventory system and UDI and HIS systems. The UDI system input can clearly recognize every medical device, rapidly identify marketed medical devices, decrease misuse by medical staff, and improve the medical quality of patients.
Hence, our hospital constructed a series of smart medical device management through the MIS inventory management system.
■System data filing:
Data is filed after changes in medical device tenders and the manufacturers are asked to come to the hospital to create images and barcodes to facilitate subsequent checks and acceptance matters according to the procedure.
■Procurement and stock management:
●E-procurement procedure:
●E-operation from procurement to goods delivery by the manufacturer is used to shorten the delivery time and decrease manual operations.
Informatized check and acceptance operation: informatized check and acceptance is carried out based on information on the “Material Procurement Form and Acceptance Check Form”. The barcode on the order form is scanned to obtain the product name, specification, model number, label, place of manufacture, medical device license number, and image. After that, the medical device product barcode is scanned again. The two bar codes must match and their information must match to complete acceptance check. The usage department will complete acceptance and implement entire process control to optimize the configuration of limited resources and improve medical device management efficiency.
■Material inventory management:
●Transparent digital management: Smart information is used to achieve transparent and digital management from procurement, purchase, ordering, acceptance check, and warehousing and ensure that inventory information is timely and accurate and the materials and accounts are consistent.
●Real-time inventory dynamics: As inventory is dynamic and instantaneous, stock and order forms can be effectively controlled. An instantaneous and accurate safe stock alert can be set up to achieve optimal stock management and decrease inactive inventory and overstock costs.
●Graphical barcode management: PDAs and mobile phones are used to display the images of goods from barcodes to effectively increase the accuracy and work performance of material distribution.
●Material counter hotspots: MIS stock acquisition big data is used to arrange suitable locations for placing goods, create goods hotspots, decrease staff movement regions, and increase work performance.
●Epidemic control material kanban: Instantaneous stock management data is used for real-time presentation of epidemic control material stock data so that the hospital can effectively control epidemic control materials.
As a logistics staff in medical institutions, the upgrading from traditional to smart system and evolution from manual identification to barcoding both follows a patient safety-centric service concept and efforts are made to maintain medical quality.
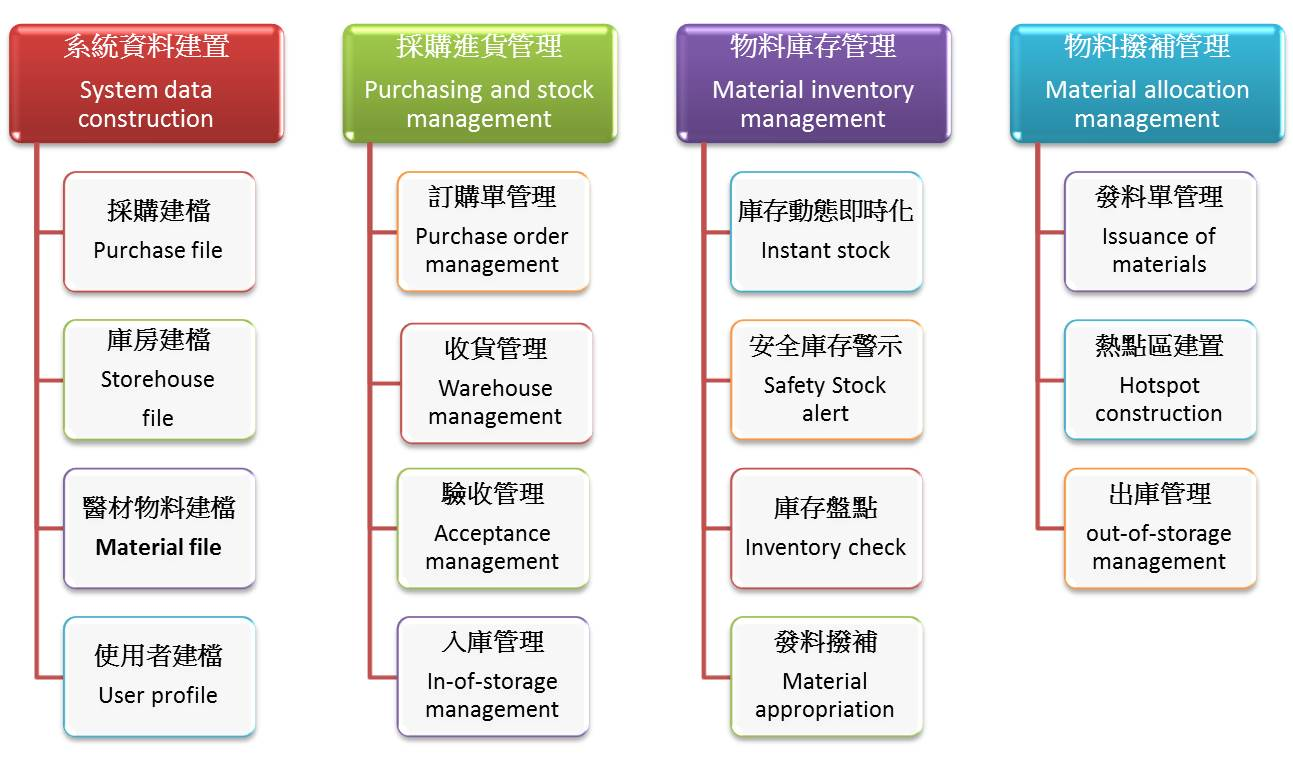
Figure 1. Medical device smart management framework
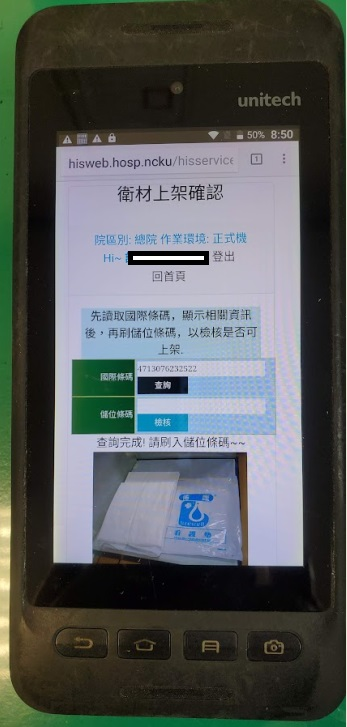
Figure 2. PDA mobile phone database management system application
